Efficacy
Testogel restores testosterone to within the eugondal range (300 - 1000 ng/dL). Normal testosterone levels were achieved at day 182 in 82.2% (139/169) of hypogonadal men treated with an optimised dose. In a randomised, placebo-controlled trial involving 274 patients (n=234 in testosterone group and n=40 in placebo group), normal testosterone levels were achieved at day 182 in 82.2% (139/169) of hypogondal men treated with an optimised dose, compared with 28.6% (8/28) of patients in the placebo group.1
Testogel restores testosterone levels to within the normal range
(Values shown in the figure below denote mean testosterone levels over a 24 h period at Day 182 of the study)
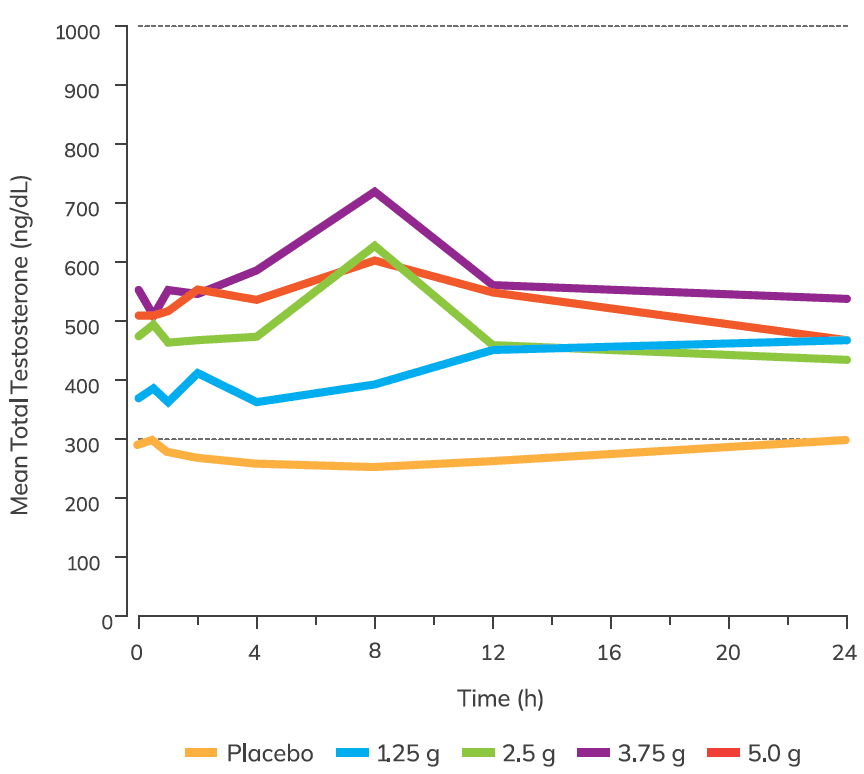
Adapted from Kaufman JM, et al.1
Mean concentration-time profiles of total testosterone. Dotted lines indicate normal testosterone range.
Study details
In a multicentre, randomised, double-blind, placebo-controlled study, the efficacy of titrated doses of Testogel was evaluated in 274 hypogonadal men compared to placebo. Aged 18–80 years with average serum total testosterone concentrations <300ng/dL (<10.4nmol/L) and PSA <2.5 ng/mL. Study population were otherwise healthy men (n=234 Testogel; n=40 placebo). Testogel (1.62%), 1.25 g, 2.5 g, 3.75 g, and 5.0 g, or placebo gel was applied once daily to either upper arms/shoulders. Dose adjustments were made on days 14, 28, and 42. Mean baseline testosterone was similar between both treatment arms (282 ng/dL (9.8nmol/L) Testogel; 294 ng/dL (10.2 nmol/L) placebo).
Following titration, significantly more participants receiving Testogel (range 65.7–82.5%) had serum total testosterone average concentration values within the eugonadal range 300 - 1000 ng/dL (10.4-34.7 nmol/L) compared with placebo (range 28.6–37.0%) across all study days (14, 56, 112, 182; p<0.0001). Normal testosterone levels were achieved in 82.2% of hypogonadal men treated with an optimised dose of Testogel at study day 182, compared to 28.6% treated with placebo (p<0.0001).
Efficacy
Following titration, significantly more participants receiving Testogel (range 65.7–82.5%) had serum total testosterone average concentration values within the eugonadal range 300–1,000 ng/dL compared with placebo (range 28.6–37.0%) across all study days (14, 56, 112, 182; p<0.0001). Normal testosterone levels were achieved in 82.2% of hypogonadal men treated with an optimised dose of Testogel at study day 182, compared to 28.6% treated with placebo (p<0.0001).
Safety information summary table1
Item | Details |
|---|---|
Study population | All 274 subjects who were randomised were included in the safety sample and there were no deaths during the study or follow-up. |
Serious treatment emergent adverse events (TEAEs) | A similar percentage of serious TEAEs occurred in the 1.62% testosterone gel (2.1%) and placebo groups (2.5%). |
Subjects with ≥1 TEAE | The percentage of subjects who experienced at least one TEAE during the study was 55.6% (130/234) for the testosterone-treated group vs. 37.5% (15/ 40) for the placebo group. |
Early Terminations Due to TEAEs | TEAEs were responsible for 25 (10.7%) early terminations in the active treatment group vs. none in the placebo group. A majority of these terminations (17/25 [68%]) were because of increased PSA, per prespecified protocol criteria (PSA >4.0 ng/mL and/or PSA increase from baseline >0.75 ng/mL). |
Cause of Most Early Terminations | Four subjects discontinued due to PSA >4 ng/mL as well as an increased velocity of >0.75 ng/mL, although upon repeat testing, they had PSA levels .0 ng/mL. The remainder was discontinued due to a PSA change of >0.75 ng/mL. Seven subjects met the criteria on the basis of a single elevated value, and although the second value did not exceed the 0.75 ng/mL criteria, the average of the two was sufficiently high to result in withdrawal. One subject, who was discontinued due to elevated PSA, was referred for biopsy. Another subject from the testosterone treatment group was diagnosed with prostate cancer during the 182-day open-label extension which was deemed possibly treatment related. Five subjects in the active treatment group had a haematocrit of >54% at some time in the study and one subject discontinued per the pre-specified protocol criteria. |
References
- 1Kaufman JM, Miller MG, Garwin JL, et al. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med. 2011;8(7):2079–2089.
Adverse event reporting
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or
Apple App Store. Adverse events should also be reported to Besins Healthcare (UK) Ltd Drug Safety on 0203 862 0920 or Email: drugsafety@besins-healthcare.com

